Management of borderline patients
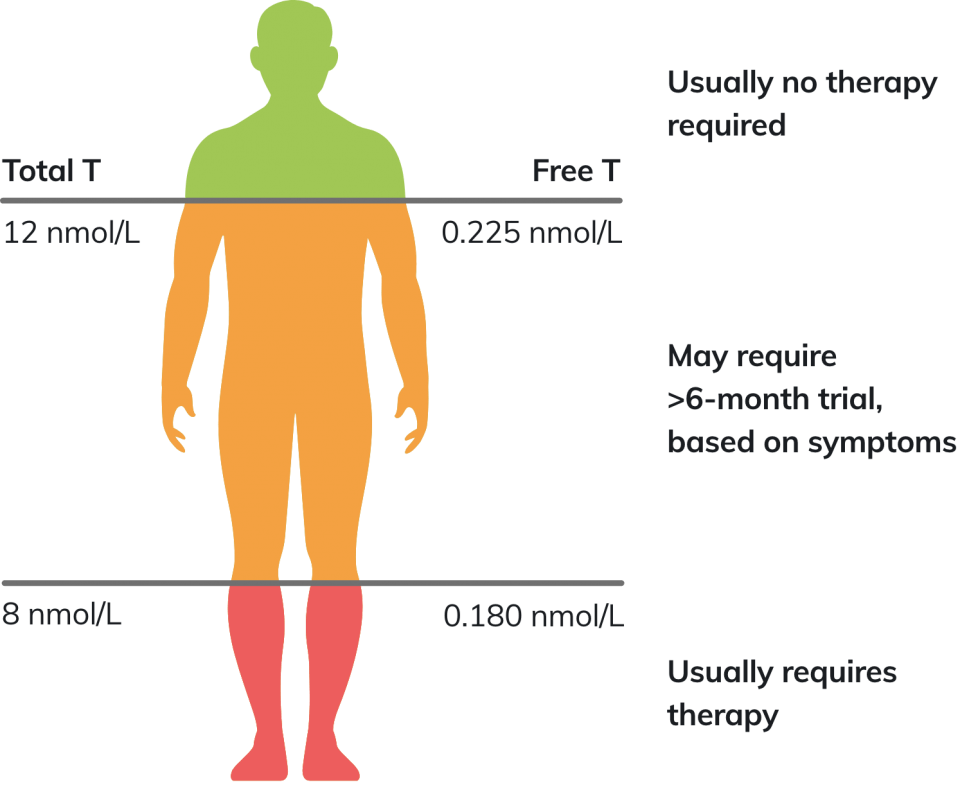
The British Society for Sexual Medicine (BSSM) and the International Society of Sexual Medicine (ISSM) recommend the following thresholds for testosterone treatment intervention in symptomatic men.
This website is provided for HCPs in the UK by Besins Healthcare (UK) Ltd.

The British Society for Sexual Medicine (BSSM) and the International Society of Sexual Medicine (ISSM) recommend the following thresholds for testosterone treatment intervention in symptomatic men.

When total testosterone (TT) levels are close to the lower normal range (8–12 nmol/L), free testosterone (FT) levels should be calculated.1
When TT level is borderline or low to normal, particularly in older men or men with obesity, sex hormone-binding globulin (SHBG) levels should be assessed (for known or suspected abnormal SHBG levels, FT also should be measured).
A FT level lower than 0.225 nmol/L provides supportive evidence for testosterone therapy (TTh), if appropriate symptoms are also present1
When TT levels are lower than 12 nmol/L, serum luteinising hormone (LH) levels should be measured to determine if it is primary or secondary testosterone deficiency (TD).1
Increased LH levels and testosterone levels below normal indicate testicular failure, so TTh should be considered1
References
TES/2022/046. February 2023.
Adverse event reporting
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Besins Healthcare (UK) Ltd Drug Safety on 0203 862 0920 or Email: pharmacovigilance@besins-healthcare.com